The Future of GMP (fGMP) in BioPharma and Life Sciences
.jpeg)
Introduction
Ensuring FDA compliance with Good Manufacturing Practices (GMP) is essential in the biopharmaceutical industry. Storage chamber failures, temperature fluctuations, and mechanical breakdowns don’t just cause operational disruptions—they threaten valuable pharmaceuticals and, most importantly, patient health. This is why innovative products, like OverShield, are game changers in this industry. Facilities no longer have to wait for a minor problem to materialize into a full-blown malfunction before they are alerted. OverShield detects and warns about these problems before they pose a threat to the chamber environment, enabling proactive maintenance that can be conducted during regular business hours. This is the future of GMP—where emergency repairs, unexpected failures, and devastating product losses are preventable.
Behind Predictive Technology
Traditional GMP monitoring systems operate in real-time, measuring temperature, pressure, humidity, and other relevant parameters. When something goes out of specification, the system issues a warning to the response team, who must rapidly troubleshoot the cause of the problem and perform repairs to protect the chamber contents—generally during off-hours. This type of monitoring is not predictive because it requires an immediate reaction to an existing condition.
Unlike monitoring-only systems, OverShield collects data and analyzes it using machine learning and AI-powered data analytics to identify trends and patterns unique to each refrigeration system. Over time, this data forms a complete profile of how each chamber responds to different events and changing conditions. With this extensive baseline of operating data, it is possible to detect subtle anomalies that would elude detection by traditional monitoring. This approach is predictive because these anomalies are often detectable before any physical parameter exceeds specification, providing valuable time to troubleshoot and schedule maintenance. This is why OverShield can see—and prevent—issues that monitoring-only solutions cannot.
Key Benefits of OverShield in GMP Compliance
Real-Time Anomaly Detection and Early Warnings
Every chamber operates differently due to unique factors ranging from age and configuration to environmental conditions. OverShield’s AI creates chamber-specific baselines and then monitors trends to identify when key metrics—such as temperature, pressure, or amperage—deviate from those standard patterns. When anomalies are detected, Predictive Monitor’s HVAC experts review the data internally to determine the appropriate level of response. When a credible threat is identified, the customer’s maintenance team is alerted via email, text, or phone with the necessary information to repair and rectify the situation.

Proactive, Tailored Maintenance Recommendations
OverShield doesn’t just notify when things go wrong; it lets you know when everything is all right. As part of the OverShield service, weekly reports are issued that summarize each chamber’s activity and generate an overall score (from 0 to 100) to provide insight and reassurance that your chambers are running optimally. The OverShield Weekly Report includes maintenance suggestions and provides information such as:
• Anomaly Descriptions
• Severity and Risk Assessments
• Root Cause Identification
• Mitigation Actions
• Weekly, monthly, and quarterly trends
With this information, teams can prioritize maintenance schedules and allocate resources to minimize chamber downtime.
Mitigation of Compliance Risks
OverShield’s AI-powered analytics prescriptively analyze each piece of refrigeration equipment with contextualized data, resulting in remarkable forewarnings and accurate maintenance insights. [See Figure 1] This predictive technology minimizes risks by continuously monitoring operating conditions for indicators of potential future malfunctions. This gives companies time to run diagnostics, schedule repairs, and keep their chambers compliant, thereby avoiding problems with audits, recalls, and fines.
Figure 1: Excerpt from an OverShield Weekly Report
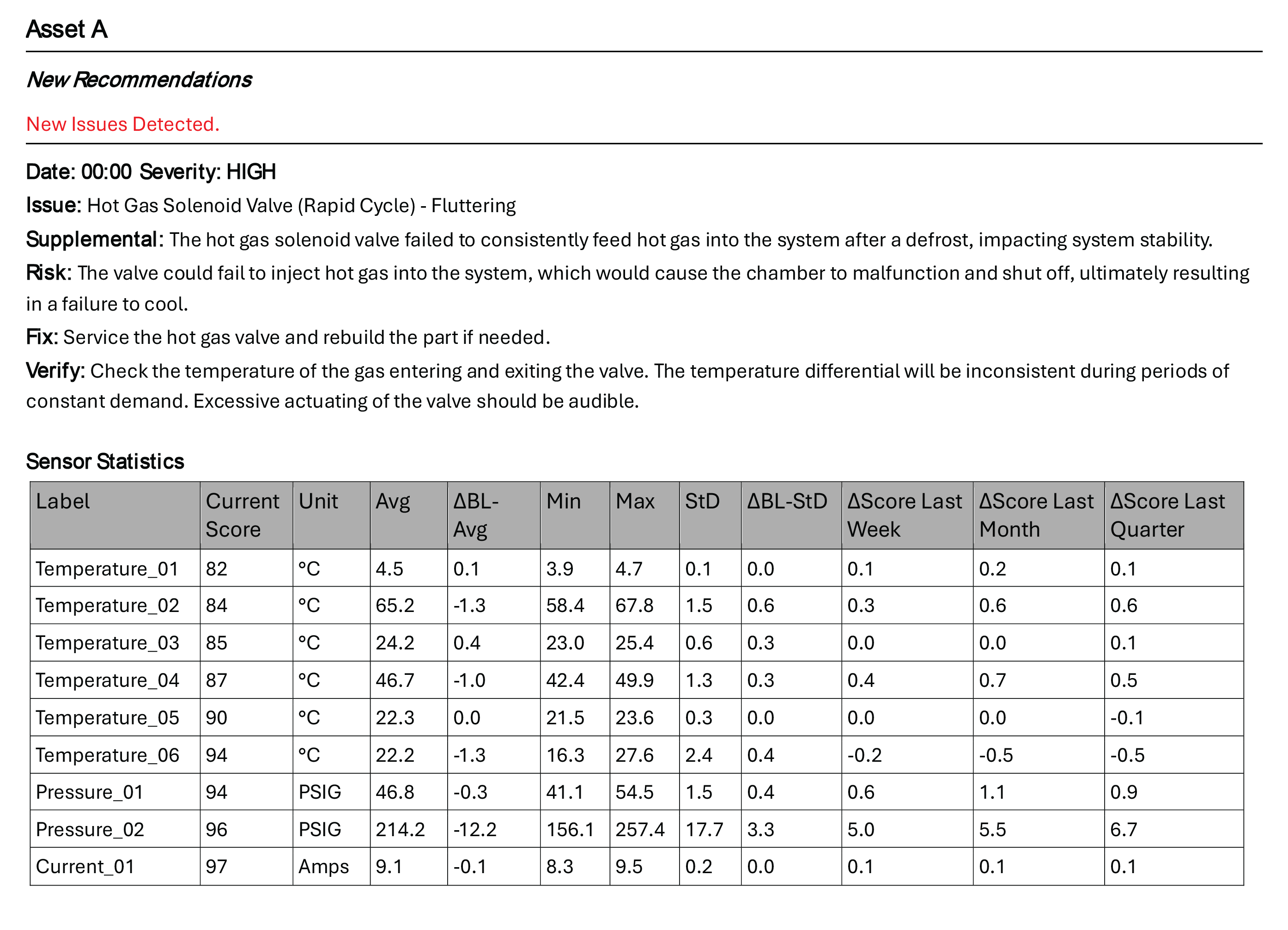
Prevent Costly Failures
Unforeseen chamber malfunctions result in substantial financial losses due to spoiled products, emergency callouts, and the halting of clinical trials and stability studies. Unlike reactive responses that disrupt schedules, OverShield’s early warnings provide the lead time needed to address underlying issues, thereby preventing catastrophic shutdowns. This proactive maintenance approach enables planned repairs during regular hours and ensures seamless workflows.
Quick ROI Through Efficiency Gains
Beyond the reduced expenditure from streamlined maintenance, reduced overtime, and fewer emergencies, OverShield also provides the necessary data to ensure that chambers are operating with optimal energy efficiency to lower operating costs. The OverShield Reports show how chambers trend from week to week, month to month, and quarter to quarter, allowing you to identify changes in performance, even as they continue to function correctly.
.jpeg)
OverShield—The Future of GMP Monitoring
AI-powered analytics systems, such as OverShield, represent future GMP (fGMP) and are essential tools for staying competitive in an increasingly challenging industry landscape. By enabling predictive maintenance, OverShield empowers GMP managers to regain control, mitigate risks, operate confidently, and deliver value rather than responding to crises.
fGMP is here. Contact Predictive MonitorSM today to discover how you can protect your refrigerated chambers, enhance operations, and ensure patient health.
See OverShield in Action
Outcomes





%20(1).jpeg)
.jpeg)














